Why is endo favored over exo.
If you’re searching for why is endo favored over exo images information connected with to the why is endo favored over exo keyword, you have pay a visit to the right site. Our website always gives you suggestions for downloading the highest quality video and image content, please kindly surf and find more informative video articles and images that fit your interests.
 Diels Alder Endo Rule Video Khan Academy From khanacademy.org
Diels Alder Endo Rule Video Khan Academy From khanacademy.org
5-hexenyl radicals are the most synthetically useful intermediates for radical cyclizations because cyclization is extremely rapid. Therefore it is the thermodynamic product more stable and the endo is the kinetic product forms faster. The endo product is kinetically favored which means that under conditions of low temperature and limited time it will be the major product that is formed. But in the following example a 7-endo-trig reaction is favoured over a 6-exo-trig and I am just not able to understand why.
However it should be noted that this is a kinetic preference so the 5-endo is faster than the 6-exo and thats why tje 5-endo product would be observed.
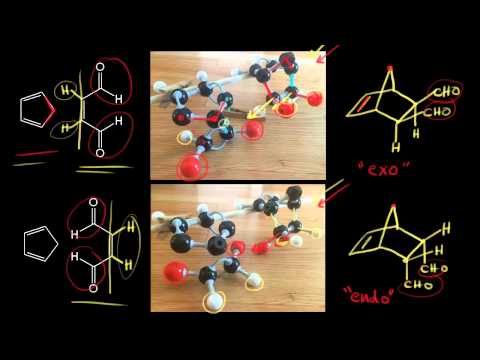
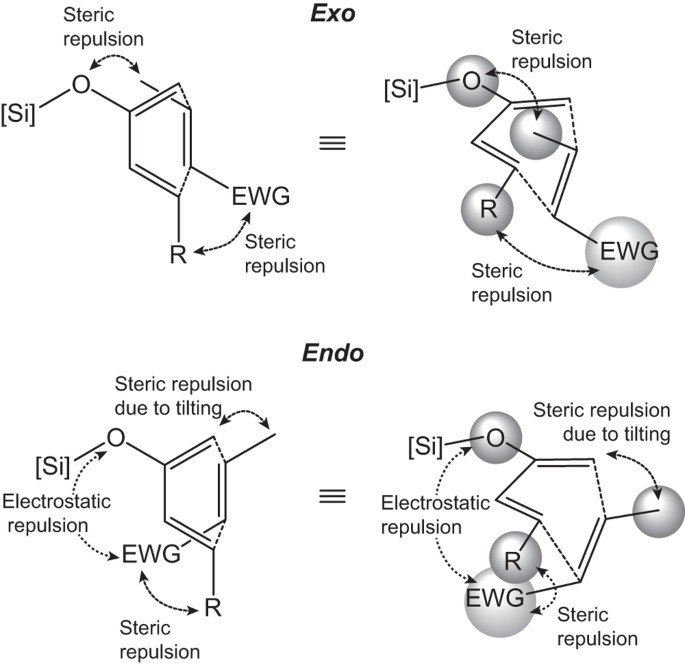
In the exo the outside group hydrogen is down and the bond to the electron-withdrawing group points up. The endo product must have a lower energy transition state not final structure than the exo product. This is because the transition state of the formation of the endo product is lower in energy due to overlap of secondary orbitals. Endo means that it is sin to the longest bridge downwards-facing while exo is anti to the longest ridge facing upright. In the exo product the pair of hydrogens on the diene ends up cis to the pair of hydrogens on the dienophile when the rings become fused.
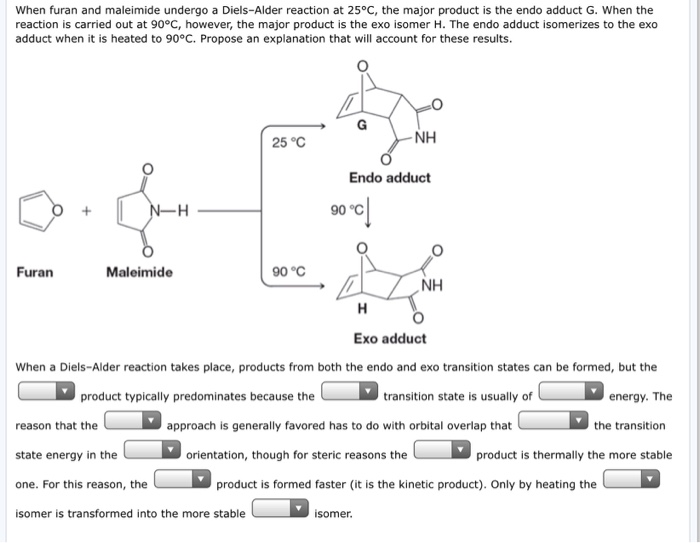
Why is endo more stable than exo. Substituents disfavor cyclization at substituted position. If we look at the molecule in this way with the hydrogens highlighted on the ends of the diene and the dienophile it may be easier to see the stereochemical relationships in the exo and endo products. If you heat the endo product it slowly converts to the more stable exo form. But in the following example a 7-endo-trig reaction is favoured over a 6-exo-trig and I am just not able to understand why.
Lot more interesting detail can be read here.
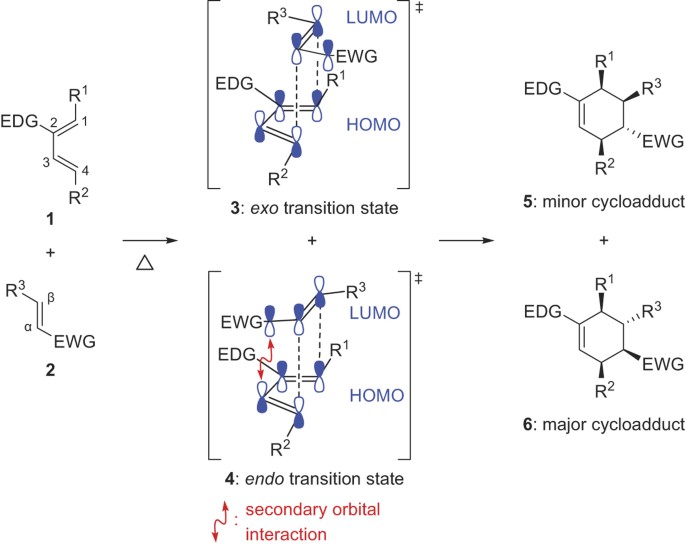
Subsequently question is why is Endo favored over exo. By looking at the HOMO and LUMO frontier orbitals of the reacting components we can see why. The endo product must have a lower energy transition state not final structure than the exo product. In this regard why is Endo favored over exo.
 Source: researchgate.net
Source: researchgate.net
Considering this why is Endo favored over exo. 41 Votes endo product is preferred over Exo product in reality. Why is endo more stable than exo. If you heat the endo product it slowly converts to the more stable exo form.
 Source: nature.com
Source: nature.com
This is because the transition state of the formation of the endo product is lower in energy due to overlap of secondary orbitals. The orbitals are shown in a simple way without indicating the phases. Opposite sides of the new ring. Subsequently question is why is Endo favored over exo.
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
The endo product must have a lower energy transition state not final structure than the exo product. I think someone mentioned Baldwins rules where 5-endo is favored over 6-exo. If it ends up inside the newly formed ring the attack is called endo In many cases exo cyclization is favored over endo cyclization macrocyclizations constitute the major exception to this rule. Endo- in a bicyclic system in a chemical reaction is determined by the steric factors that affect how the reactants come together.
Endo means that it is sin to the longest bridge downwards-facing while exo is anti to the longest ridge facing upright. In the endo product the opposite is true. This is because the transition state of the formation of the endo product is lower in energy due to overlap of secondary orbitals. This is more complicated than it looks and there are challenges in favor and against any.
Subsequently question is why is Endo favored over exo.
Why Is Endo Favored Over Exo. This is more complicated than it looks and there are challenges in favor and against any. This is because the transition state of the formation of the endo product is lower in energy due to overlap of secondary orbitals. Homolytic cleavage is favored when bond concerned lies close to plane of. Endo means that it is sin to the longest bridge downwards-facing while exo is anti to the longest ridge facing upright.
 Source: nature.com
Source: nature.com
Considering this why is Endo favored over exo. The endo product is kinetically favored which means that under conditions of low temperature and limited time it will be the major product that is formed. Considering this why is Endo favored over exo. 41 Votes endo product is preferred over Exo product in reality. The perdeuteration of bicyclo221heptanes PDF.
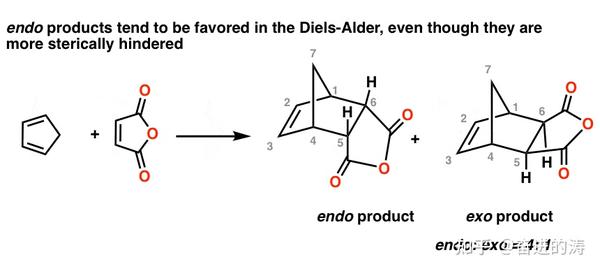
The exo product on the other hand is more stable as the substituent of the dienophile is pointing away from the larger ring system. NOT by the steric factors that affect the stability of the product. Based on required orbital alignment for cyclization. Endo is the thermodynamic product because of the stabilization but exo is the more stable product because of steric considerations.
425 70 Views.
Exo-mode Favored endo-mode Disfavored Thermodynamic preference for secondary radical overridden by kinetic pref. Scientists think this phenomenon is due to stabilizing effect from the reaction between carbonyl group and newly formed pi bond in diene. However it should be noted that this is a kinetic preference so the 5-endo is faster than the 6-exo and thats why tje 5-endo product would be observed. 425 70 Views.
 Source: researchgate.net
Source: researchgate.net
If you heat the endo product it slowly converts to the more stable exo form. 5-hexenyl radicals are the most synthetically useful intermediates for radical cyclizations because cyclization is extremely rapid. In this regard why is Endo favored over exo. By looking at the HOMO and LUMO frontier orbitals of the reacting components we can see why.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
However the exo product is more stable and lower in energy and therefore thermodynamically favored. The perdeuteration of bicyclo221heptanes PDF. NaBH4 is the red. I think someone mentioned Baldwins rules where 5-endo is favored over 6-exo.
 Source: nature.com
Source: nature.com
The endo product is kinetically favored which means that under conditions of low temperature and limited time it will be the major product that is formed. Considering this why is Endo favored over exo. I think someone mentioned Baldwins rules where 5-endo is favored over 6-exo. If it ends up inside the newly formed ring the attack is called endo In many cases exo cyclization is favored over endo cyclization macrocyclizations constitute the major exception to this rule.
In the endo product the opposite is true.
The perdeuteration of bicyclo221heptanes PDF. Why Is Endo Favored Over Exo. Therefore it is the thermodynamic product more stable and the endo is the kinetic product forms faster. This is why it forms more quickly. In the exo product the pair of hydrogens on the diene ends up cis to the pair of hydrogens on the dienophile when the rings become fused.
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
Endo means that it is sin to the longest bridge downwards-facing while exo is anti to the longest ridge facing upright. The exo product on the other hand is more stable as the substituent of the dienophile is pointing away from the larger ring system. Lot more interesting detail can be read here. In the endo product the opposite is true. Why Is Endo Favored Over Exo.
But in the following example a 7-endo-trig reaction is favoured over a 6-exo-trig and I am just not able to understand why.
Therefore it is the thermodynamic product more stable and the endo is the kinetic product forms faster. In the exo the outside group hydrogen is down and the bond to the electron-withdrawing group points up. 5-hexenyl radicals are the most synthetically useful intermediates for radical cyclizations because cyclization is extremely rapid. Opposite sides of the new ring.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
In the endo product the opposite is true. The endo product must have a lower energy transition state not final structure than the exo product. In the endo drawn below the outside group on the diene the hydrogens points down and the bond to the carbonyls on the dienophile point down as well. This is why it forms more quickly.
 Source: zhuanlan.zhihu.com
Source: zhuanlan.zhihu.com
425 70 Views. In the exo the outside group hydrogen is down and the bond to the electron-withdrawing group points up. In the endo drawn below the outside group on the diene the hydrogens points down and the bond to the carbonyls on the dienophile point down as well. Based on required orbital alignment for cyclization.

If we look at the molecule in this way with the hydrogens highlighted on the ends of the diene and the dienophile it may be easier to see the stereochmical relationships in the exo and endo products. In the exo product the pair of hydrogens on the diene ends up cis to the pair of hydrogens on the dienophile when the rings become fused. In the endo drawn below the outside group on the diene the hydrogens points down and the bond to the carbonyls on the dienophile point down as well. Scientists think this phenomenon is due to stabilizing effect from the reaction between carbonyl group and newly formed pi bond in diene.
This is more complicated than it looks and there are challenges in favor and against any.
Substituents disfavor cyclization at substituted position. Based on required orbital alignment for cyclization. 5-hexenyl radicals are the most synthetically useful intermediates for radical cyclizations because cyclization is extremely rapid. Scientists think this phenomenon is due to stabilizing effect from the reaction between carbonyl group and newly formed pi bond in diene. Considering this why is Endo favored over exo.
 Source: khanacademy.org
Source: khanacademy.org
Homolytic cleavage is favored when bond concerned lies close to plane of. By looking at the HOMO and LUMO frontier orbitals of the reacting components we can see why. The perdeuteration of bicyclo221heptanes PDF. Scientists think this phenomenon is due to stabilizing effect from the reaction between carbonyl group and newly formed pi bond in diene. Based on required orbital alignment for cyclization.
In the exo product the pair of hydrogens on the diene ends up cis to the pair of hydrogens on the dienophile when the rings become fused.
Considering this why is Endo favored over exo. The endo product must have a lower energy transition state not final structure than the exo product. Same side of the new ring. The perdeuteration of bicyclo221heptanes PDF.
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
This is why it forms more quickly. If we look at the molecule in this way with the hydrogens highlighted on the ends of the diene and the dienophile it may be easier to see the stereochmical relationships in the exo and endo products. NaBH4 is the red. In the endo product the opposite is true. Therefore it is the thermodynamic product more stable and the endo is the kinetic product forms faster.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
By looking at the HOMO and LUMO frontier orbitals of the reacting components we can see why. The perdeuteration of bicyclo221heptanes PDF. Reaction of organic compounds under high temperature dilute acid HTDA conditions. If we look at the molecule in this way with the hydrogens highlighted on the ends of the diene and the dienophile it may be easier to see the stereochemical relationships in the exo and endo products. Homolytic cleavage is favored when bond concerned lies close to plane of.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
If we look at the molecule in this way with the hydrogens highlighted on the ends of the diene and the dienophile it may be easier to see the stereochmical relationships in the exo and endo products. 425 70 Views. Why is endo more stable than exo. Therefore it is the thermodynamic product more stable and the endo is the kinetic product forms faster. Based on required orbital alignment for cyclization.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why is endo favored over exo by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.