Endo and exo isomers examples.
If you’re searching for endo and exo isomers examples images information linked to the endo and exo isomers examples keyword, you have visit the right blog. Our site always provides you with suggestions for downloading the highest quality video and picture content, please kindly search and find more informative video content and graphics that match your interests.
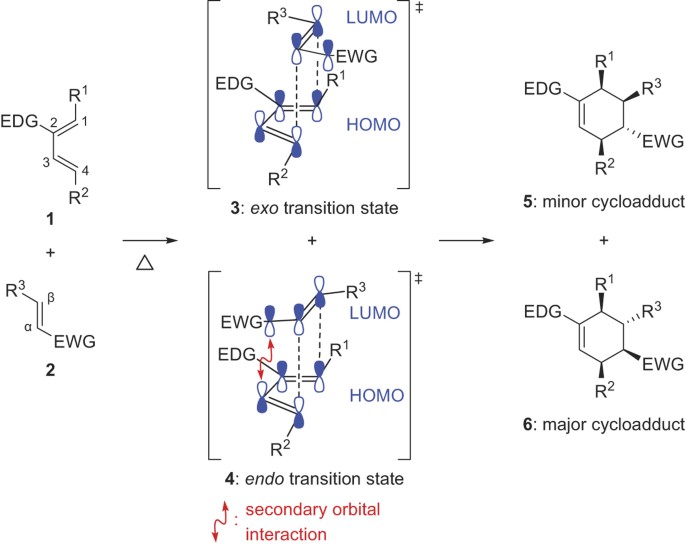 Unconventional Exo Selectivity In Thermal Normal Electron Demand Diels Alder Reactions Scientific Reports From nature.com
Unconventional Exo Selectivity In Thermal Normal Electron Demand Diels Alder Reactions Scientific Reports From nature.com
Two examples follow which are drawn to emphasize how suprafacial addition occurs. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring. The endo and exo products are really two different diastereomers.
Beta and Y-type zeolites exhibit higher activity because of their large three-dimensional channels.
Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. Translations in context of endo in English-German from Reverso Context. 3 This is because of the interaction and coupling with the H-5 and H-6 as displayed in Figure 1. Surface passivation of Hβ confirms that the reaction proceeds in the inner channels. Organic chemistry An isomer of a bridged organic compound in which a particular substituent is located closest to the.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
Since each chiral centre could have two possible configurations there are sixteen possible stereoisomers that could result in the reaction shown above. What does endo-isomer mean. A peak at 300 ppm would appear in the exo isomer spectra as opposed to a peak at 360 ppm as shown in the observed endo product. And is orientated towards the lowest numbered. The actual product formed is the endo adduct.
The prefix exo is reserved for the isomer with the substituent located closest or syn to the shortest bridge.
If it is orientated away from the highest numbered. Two examples follow which are drawn to emphasize how suprafacial addition occurs. Exothermic and endothermic reactions When a chemical reaction occurs energy is transferred to or from the surroundings. The endo- to exo-isomerization of dicyclopentadiene was performed in liquid phase using acidic zeolites.
 Source: j-tradition.com
Source: j-tradition.com
The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring. This video contains plenty of examples and practice problems. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to. Among the zeolites tested the activity order is Hβ HY HUSY HZSM-5 H-mordenite. The endo and exo products are really two different diastereomers. What does endo-isomer mean.
 Source: j-tradition.com
Source: j-tradition.com
The actual product formed is the endo adduct. In the first example dimethyl cis -butadioate adds to 13-butadiene to give a cis-substituted cyclohexene. Translations in context of endo in French-English from Reverso Context. In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring.
This video contains plenty of examples and practice problems. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts. This video also helps you to see when you get 1 or 2 different constitutional isomers.
The ENDO product is the one where the outside groups on the diene are on the SAME side of the 6-membered ring as the electron withdrawing group EWG.
A peak at 300 ppm would appear in the exo isomer spectra as opposed to a peak at 360 ppm as shown in the observed endo product. The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts. The exo isomer would possess a triplet around 350 ppm due to the difference in dihedral angle between the hydrogen molecules of H-1 and H-4 and H-5 and H-6 Figure 1. Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. Here longest and shortest refer to the.
 Source: j-tradition.com
Source: j-tradition.com
The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products the endo and the exo adducts. If it is orientated away from the highest numbered. The stereochemistry of the products are also discussed such as the cis and trans diastereomer isomers that can be formed in addition to enantiomers and meso compounds. The actual product formed is the endo adduct. C-7 in example below it is given the description exo.
This video contains plenty of examples and practice problems. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge. Surface passivation of Hβ confirms that the reaction proceeds in the inner channels. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol.
Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system.
It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. If the group is attached to the highest numbered. The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to. The ENDO product is the one where the outside groups on the diene are on the SAME side of the 6-membered ring as the electron withdrawing group EWG. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer. There is usually a temperature change. For example when a bonfire burns it. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene.
 Source: j-tradition.com
Source: j-tradition.com
This video contains plenty of examples and practice problems. Here longest and shortest refer to the number of atoms that. If you think about it you can see that when two rings fuse together to make a third four new stereocenters can be created. The prefix exo is reserved for the isomer with the substituent located closest or syn to the shortest bridge.
In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring.
What does endo-isomer mean. The ENDO product is the one where the outside groups on the diene are on the SAME side of the 6-membered ring as the electron withdrawing group EWG. This is because although the hydrogens of the maleic anhydride must be cis in the product there are two possible arrangements where this is true. Since each chiral centre could have two possible configurations there are sixteen possible stereoisomers that could result in the reaction shown above. Beta and Y-type zeolites exhibit higher activity because of their large three-dimensional channels.
 Source: j-tradition.com
Source: j-tradition.com
The endo and exo products are really two different diastereomers. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge. 3 This is because of the interaction and coupling with the H-5 and H-6 as displayed in Figure 1. Translations in context of endo in English-German from Reverso Context. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to.
The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge.
For example when a bonfire burns it. Exothermic and endothermic reactions When a chemical reaction occurs energy is transferred to or from the surroundings. There is usually a temperature change. If the group is attached to the highest numbered.
 Source: j-tradition.com
Source: j-tradition.com
In the second example suprafacial approach of a dienophile to the 25 carbons of transtrans -24-hexadiene is seen to lead to a product with two methyl groups on the same side of the cyclohexene ring. Two examples follow which are drawn to emphasize how suprafacial addition occurs. This video also helps you to see when you get 1 or 2 different constitutional isomers. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
If it is orientated away from the highest numbered. It is possible to convert the endo isomer 5b to the exo isomer 5a by an epimerization process through the enol. For example when a bonfire burns it. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge.
 Source: nature.com
Source: nature.com
The endo- to exo-isomerization of dicyclopentadiene was performed in liquid phase using acidic zeolites. The actual product formed is the endo adduct. There is usually a temperature change. Beta and Y-type zeolites exhibit higher activity because of their large three-dimensional channels.
The prefix exo is reserved for the isomer with the substituent located farthest or anti to the longest bridge.
The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. The actual product formed is the endo adduct. A detergent comprising tetrahydrodicyclopentadiene in which the endoexo isomer ratio by weight is from 7030 to 0100. Since each chiral centre could have two possible configurations there are sixteen possible stereoisomers that could result in the reaction shown above.
 Source: j-tradition.com
Source: j-tradition.com
Translations in context of endo in English-German from Reverso Context. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge. C-7 in example below it is given the description exo. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to.
And is orientated towards the lowest numbered.
This is tentatively assigned as the endo isomer and on base treatment it isomerizes to a new compound identified as the exo isomer. C-7 in example below it is given the description exo. The endo and exo products are really two different diastereomers. The prefix endo is reserved for the isomer with the substituent located closest or syn to the longest bridge.
 Source: j-tradition.com
Source: j-tradition.com
For example when a bonfire burns it. Here longest and shortest refer to the number of atoms that. Here longest and shortest refer to the. The actual product formed is the endo adduct. The exo isomer would possess a triplet around 350 ppm due to the difference in dihedral angle between the hydrogen molecules of H-1 and H-4 and H-5 and H-6 Figure 1.
 Source: europepmc.org
Source: europepmc.org
There is usually a temperature change. This diastereoisomer is the less stable one but is formed. In the first example dimethyl cis -butadioate adds to 13-butadiene to give a cis-substituted cyclohexene. Translations in context of endo in English-German from Reverso Context. There is usually a temperature change.
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. Surface passivation of Hβ confirms that the reaction proceeds in the inner channels. Endoexo isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. Giga-fren In sharp contrast to their 321024 analogs the endo isomer is stable while the exo readily rearranges to. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title endo and exo isomers examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





