Diels alder exo endo.
If you’re searching for diels alder exo endo images information connected with to the diels alder exo endo topic, you have visit the ideal blog. Our website always provides you with hints for seeking the highest quality video and picture content, please kindly hunt and locate more informative video articles and images that fit your interests.
 Chapter 16 17 Discussion Endo And Exo Are Meaningless Without Substitutents To Provide Frame Of Reference Meso Identical Ppt Download From slideplayer.com
Chapter 16 17 Discussion Endo And Exo Are Meaningless Without Substitutents To Provide Frame Of Reference Meso Identical Ppt Download From slideplayer.com
Diels-Alder orbital explanation for the endo rule. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring. 20 models in this collection.
20 models in this collection.
The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. This reaction was first made by Breslow and Oda. The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring.
 Source: slideplayer.com
Source: slideplayer.com
There is not a single mechanism for all Diels-Alder reactions. 20 models in this collection. However the DielsAlder is a reversible reaction. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11.
The Diels Alder reaction is probably the most common cycloaddition.
The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. Reactions Under Orbital Control. When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed. As you might predict the exo position refers to the outside position.
 Source: youtube.com
Source: youtube.com
It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. As you might predict the exo position refers to the outside position. The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11.
 Source: pinterest.com
Source: pinterest.com
In most cases the endo product is consid. In a recent publication it was proposed that this exo preference is due to a thremodynamic effect. 20 models in this collection. This looks ordinary until we draw the product from a side view which reveals some nice structures and interesting features of the mechanism that leads to the formation of two stereoisomers.
 Source: pinterest.com
Source: pinterest.com
The Diels-Alder reaction is a thermal cycloaddition whose mechanism involves the sigma-overlap of the pi-orbitals of the two unsaturated systems. In the Diels-Alder reaction the Endo and Exo products are formed when a cyclic diene is reacted with a dienophile. The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring. This is why it forms more quickly.
As you might predict the exo position refers to the outside position. Endo and Exo Products. Diels-Alder orbital explanation for the endo rule. The rate at which a Diels-Alder reaction takes place depends on electronic as.
The DielsAlder reaction leads to a mixture of two diastereomers one called endo and the other one exo.
It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets. The rate at which a Diels-Alder reaction takes place depends on electronic as. Often there are already rings in the molecules undergoing reaction and a. The Diels Alder reaction is probably the most common cycloaddition. There is not a single mechanism for all Diels-Alder reactions.
 Source: slideplayer.com
Source: slideplayer.com
A The expected endo 9 and exo 10 adducts formed from the substrate analogue 8 by the spontaneous or enzyme-catalysed DielsAlder reaction. The DielsAlder reaction leads to a mixture of two diastereomers one called endo and the other one exo. Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets. In most cases the endo product is consid.
The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring. However the DielsAlder is a reversible reaction. It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system.
This is why it forms more quickly.
The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. In this lecture I have discussed the Diels-Alder reaction and selectivity for endo or exo product. However the DielsAlder is a reversible reaction. In the Diels-Alder reaction the Endo and Exo products are formed when a cyclic diene is reacted with a dienophile.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. Exo And Endo Diels-Alder Products Are Diastereomers Of Each Other 3-D Models Of The Endo And Exo Products In The Diels-Alder Between Cyclopentadiene And Maleic Anhydride Distinguishing Endo vs Exo. Often there are already rings in the molecules undergoing reaction and a. The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring.
 Source: pinterest.com
Source: pinterest.com
The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11. The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11. When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed. Often there are already rings in the molecules undergoing reaction and a.
 Source: youtube.com
Source: youtube.com
The rate at which a Diels-Alder reaction takes place depends on electronic as. This reaction was first made by Breslow and Oda. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system.
A The expected endo 9 and exo 10 adducts formed from the substrate analogue 8 by the spontaneous or enzyme-catalysed DielsAlder reaction.
The Diels-Alder reaction is a thermal cycloaddition whose mechanism involves the sigma-overlap of the pi-orbitals of the two unsaturated systems. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. There is not a single mechanism for all Diels-Alder reactions. It allows the construction of six-membered rings which are very common in biological small molecules which are frequently synthetic targets.
 Source: pinterest.com
Source: pinterest.com
I have explained the complete mechanism with the help. 20 models in this collection. In the Diels-Alder reaction the Endo and Exo products are formed when a cyclic diene is reacted with a dienophile. The Diels Alder reaction is probably the most common cycloaddition. This looks ordinary until we draw the product from a side view which reveals some nice structures and interesting features of the mechanism that leads to the formation of two stereoisomers.
It turns out that the rate of formation of the expected endo product is actually 500 times faster than the rate of formation of the exo product.
Endo and Exo Products. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. This is why it forms more quickly. The endo product must have a lower energy transition state not final structure than the exo product.
 Source: pinterest.com
Source: pinterest.com
Endo and Exo products of Diels-Alder Reaction with Practice Problems - Chemistry Steps. I have explained the complete mechanism with the help. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. Often there are already rings in the molecules undergoing reaction and a.
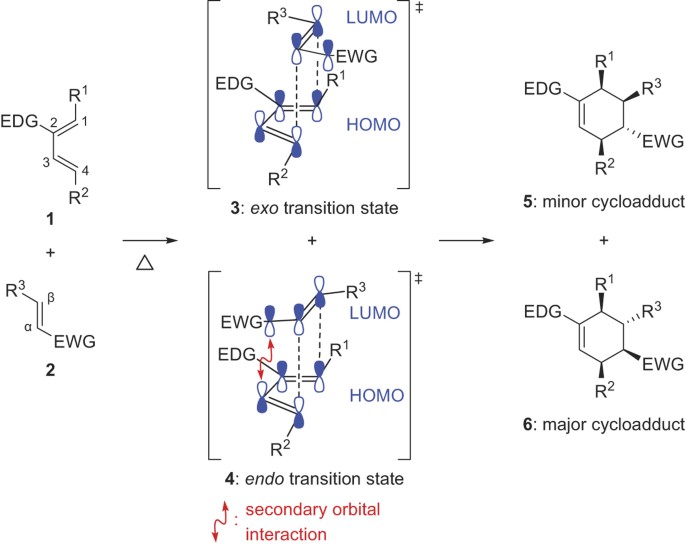 Source: nature.com
Source: nature.com
This is why it forms more quickly. The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger six-membered ring. The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. Use getProperty modelInfo or getProperty auxiliaryInfo to inspect them.
 Source: pinterest.com
Source: pinterest.com
Endo and Exo products of Diels-Alder Reaction with Practice Problems - Chemistry Steps. Exo And Endo Diels-Alder Products Are Diastereomers Of Each Other 3-D Models Of The Endo And Exo Products In The Diels-Alder Between Cyclopentadiene And Maleic Anhydride Distinguishing Endo vs Exo. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. In a recent publication it was proposed that this exo preference is due to a thremodynamic effect.
However the DielsAlder is a reversible reaction.
The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. The Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran yields only the exo adduct Figure 11. Endo and Exo products of Diels-Alder Reaction with Practice Problems - Chemistry Steps. The Diels-Alder reaction is a thermal cycloaddition whose mechanism involves the sigma-overlap of the pi-orbitals of the two unsaturated systems.
 Source: pinterest.com
Source: pinterest.com
However the DielsAlder is a reversible reaction. This reaction was first made by Breslow and Oda. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. In this lecture I have discussed the Diels-Alder reaction and selectivity for endo or exo product. A The expected endo 9 and exo 10 adducts formed from the substrate analogue 8 by the spontaneous or enzyme-catalysed DielsAlder reaction.
The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder.
Endo and Exo products of Diels-Alder Reaction with Practice Problems - Chemistry Steps. The Diels Alder reaction is probably the most common cycloaddition. When a cyclic diene is used in the Diels-Alder reaction a bridged bicyclic compound is formed. A The expected endo 9 and exo 10 adducts formed from the substrate analogue 8 by the spontaneous or enzyme-catalysed DielsAlder reaction.
 Source: pinterest.com
Source: pinterest.com
The cyclo-reversion temperature of the first one is lower than the exo adduct and the ratio between endo and exo adducts varies according to the substituents of the DielsAlder. There is not a single mechanism for all Diels-Alder reactions. I have explained the complete mechanism with the help. In this case the exo product is thermodynamically favored over the endo product by about 19 k c a l m o l. Exo And Endo Diels-Alder Products Are Diastereomers Of Each Other 3-D Models Of The Endo And Exo Products In The Diels-Alder Between Cyclopentadiene And Maleic Anhydride Distinguishing Endo vs Exo.
 Source: pinterest.com
Source: pinterest.com
In the Diels-Alder reaction the Endo and Exo products are formed when a cyclic diene is reacted with a dienophile. Synchronous and symmetrical concerted mechanisms when. The authors Cordes de Gala and Berson compare this exo-endo transformation to another rearrangement on the cyclopropanone shown on. The rate at which a Diels-Alder reaction takes place depends on electronic as. In most cases the endo product is consid.
 Source: pinterest.com
Source: pinterest.com
Pay Attention To The Relationship Between The Outside Groups On The Diene And The EWG On The Dienophile. In this lecture I have discussed the Diels-Alder reaction and selectivity for endo or exo product. Diels-Alder orbital explanation for the endo rule. In most cases the endo product is consid. As you might predict the exo position refers to the outside position.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title diels alder exo endo by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





