Diels alder endo vs exo.
If you’re looking for diels alder endo vs exo images information related to the diels alder endo vs exo topic, you have come to the right site. Our site frequently gives you suggestions for refferencing the highest quality video and picture content, please kindly search and find more enlightening video articles and graphics that match your interests.
 Diels Alder Reaction Of Pericyclic Reactions Dienes And Dienophiles Ryosuke University From j-tradition.com
Diels Alder Reaction Of Pericyclic Reactions Dienes And Dienophiles Ryosuke University From j-tradition.com
When Diels and Alder originally discovered this phenomenon they assigned the name endo to the major product where the dienophile is pointing in towards the alkene and the term exo outside such as in exoskeleton to refer to the minor product where the dienophile is pointing out away from the alkene. Th formation of exo vs endo is a case of kinetic vs. Fixed conformation in bicyclic products. Endo-Exo isomerism is a special type of isomerism that falls into the category of stereoisomers.
Endo and exo isomer have different dissociation temperatures and the ratio between them plays a crucial role in harnessing DA networks thermomechanical behaviors.
The exo product is more stable but the activation energy for endo is lower so the less stable endo product is formed faster. Maleic anhydride and cyclopentadiene yield the endo product in a Diels-Alder reaction though for steric reasons the exo product is thermally the more stable one. Diels-Alder Transition State Benzene The diene must adopt an s-cis conformation to be reactive. The exo product is more stable but the activation energy for endo is lower so the less stable endo product is formed faster. Qualitative and quantitative MO methods were used to test the assumption that the endo-product is the predicted kinetic product and the exo-adduct is the predicted thermodynamic product for the Diels-Alder reaction of maleic anhydride with cyclopentadiene and with furan.
 Source: exo2020reborn.blogspot.com
Source: exo2020reborn.blogspot.com
Generally the endo rule applies to Diels-Alder reactions but it is less adhered to than facial selectivity. Endo-Exo isomerism is a special type of isomerism that falls into the category of stereoisomers. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. The Diels-Alder reaction is a reversible reaction. Interactive 3D animations to predict stereochemistry of endo product of Diels-Alder reaction for students studying University courses.
When Diels and Alder originally discovered this phenomenon they assigned the name endo to the major product where the dienophile is pointing in towards the alkene and the term exo outside such as in exoskeleton to refer to the minor product where the dienophile is pointing out away from the alkene.
The Diels-Alder reaction is a reversible reaction. Qualitative and quantitative MO methods were used to test the assumption that the endo-product is the predicted kinetic product and the exo-adduct is the predicted thermodynamic product for the Diels-Alder reaction of maleic anhydride with cyclopentadiene and with furan. The exo product is more stable but the activation energy for endo is lower so the less stable endo product is formed faster. There are different ways the two original rings can combine leading to different stereochemical outcomes.
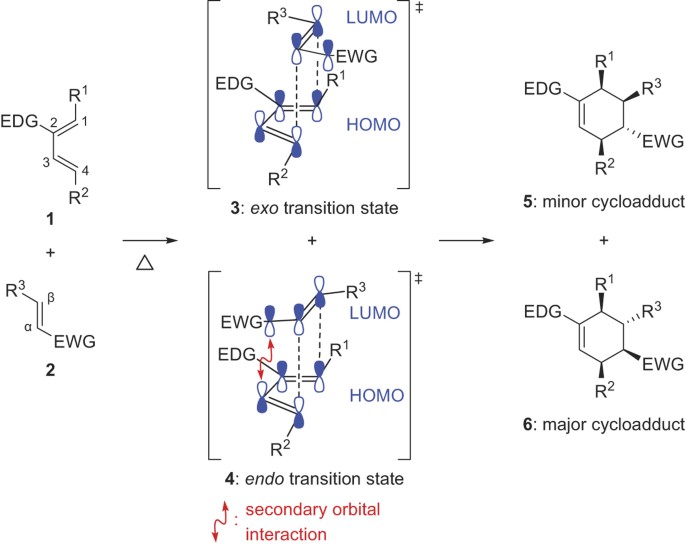 Source: nature.com
Source: nature.com
Maleic anhydride and cyclopentadiene yield the endo product in a Diels-Alder reaction though for steric reasons the exo product is thermally the more stable one. Exo isomeric attachments in Diels-Alder DA polymer networks. We will discuss thermodynamic and kinetic aspects of endo vs. The reactions were then carried out and the products analyzed and the results considered in terms of the relative energies of the reactants and products of each process and.
 Source: j-tradition.com
Source: j-tradition.com
Enjoy the videos and music you love upload original content and share it all with friends family and the world on YouTube. Exo -CHO in the Diels-Alder addition of a trans -αβ-unsaturated aldehyde to cyclopentadiene can be changed from 12 to 81 depending on temperature and BF 3. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. Hi How do I design a reaction definition that obeys the stereospecifity rules of the Diels-Alder reaction namely that the stereochemistry of the dienophile is preserved in the product and that the out groups on the termini of the diene end up cis in the product.
 Source: j-tradition.com
Source: j-tradition.com
Qualitative and quantitative MO methods were used to test the assumption that the endo-product is the predicted kinetic product and the exo-adduct is the predicted thermodynamic product for the Diels-Alder reaction of maleic anhydride with cyclopentadiene and with furan. The key difference between Endo and Exo Diels Alder compounds is that the Endo Diels Alder product has its substituents on the same face of the bridged ring system whereas the Exo Diels Alder product has its substituents on the opposite faces of the bridged ring system. Generally the endo rule applies to Diels-Alder reactions but it is less adhered to than facial selectivity. The exo product is more stable but the activation energy for endo is lower so the less stable endo product is formed faster.
The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. Diels-Alder Transition State Benzene The diene must adopt an s-cis conformation to be reactive. Generally the endo rule applies to Diels-Alder reactions but it is less adhered to than facial selectivity.
Only by heating to 200 C is the endo isomer transformed into the more stable exo isomer.
Only by heating to 200 C is the endo isomer transformed into the more stable exo isomer. The difference between endothermic and exothermic reactions lies in the words themselves. Endo and exo isomer have different dissociation temperatures and the ratio between them plays a crucial role in harnessing DA networks thermomechanical behaviors. Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. When two cyclic structures combine in a Diels Alder reaction a third ring is formed in between the original ones.
 Source: nature.com
Source: nature.com
Endo-Exo isomerism is a special type of isomerism that falls into the category of stereoisomers. Thermic refers to heat just as in the word thermometer Exo means outside and endo means inside Thus an endothermic reaction pulls heat into an. Interactive 3D animations of DA - reaction cyclopentadiene and maleic anhydride to give endo adduct for students studying University courses. Generally the endo rule applies to Diels-Alder reactions but it is less adhered to than facial selectivity. Qualitative and quantitative MO methods were used to test the assumption that the endo-product is the predicted kinetic product and the exo-adduct is the predicted thermodynamic product for the Diels-Alder reaction of maleic anhydride with cyclopentadiene and with furan.
When two cyclic structures combine in a Diels Alder reaction a third ring is formed in between the original ones. Interactive 3D animations to predict stereochemistry of endo product of Diels-Alder reaction for students studying University courses. Fixed conformation in bicyclic products. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level.
H H H H exo endo minor major.
Hi How do I design a reaction definition that obeys the stereospecifity rules of the Diels-Alder reaction namely that the stereochemistry of the dienophile is preserved in the product and that the out groups on the termini of the diene end up cis in the product. The Diels-Alder reaction is a reversible reaction. There are different ways the two original rings can combine leading to different stereochemical outcomes. Enjoy the videos and music you love upload original content and share it all with friends family and the world on YouTube.
 Source: j-tradition.com
Source: j-tradition.com
The exo product is more stable but the activation energy for endo is lower so the less stable endo product is formed faster. We will discuss thermodynamic and kinetic aspects of endo vs. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. Only by heating to 200 C is the endo isomer transformed into the more stable exo isomer.
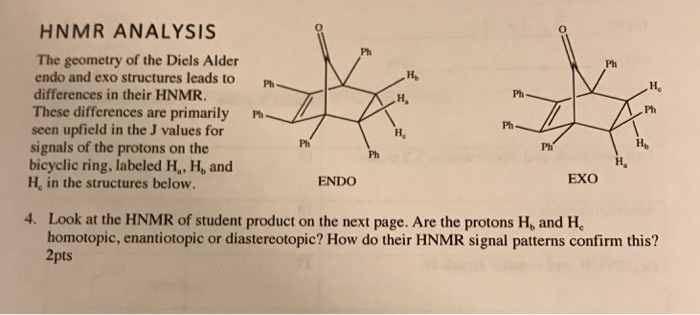
The exo product is more stable but the activation energy for endo is lower so the less stable endo product is formed faster. An exo addition looks something like this schematically. Endo and exo isomer have different dissociation temperatures and the ratio between them plays a crucial role in harnessing DA networks thermomechanical behaviors. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Generally the endo transition state is favored. Exo -CHO in the Diels-Alder addition of a trans -αβ-unsaturated aldehyde to cyclopentadiene can be changed from 12 to 81 depending on temperature and BF 3. Endo-Exo isomerism is a special type of isomerism that falls into the category of stereoisomers. Maleic anhydride and cyclopentadiene yield the endo product in a Diels-Alder reaction though for steric reasons the exo product is thermally the more stable one.
Endo-Exo isomerism is a special type of isomerism that falls into the category of stereoisomers.
Generally the endo rule applies to Diels-Alder reactions but it is less adhered to than facial selectivity. Generally the endo rule applies to Diels-Alder reactions but it is less adhered to than facial selectivity. Exo isomeric attachments in Diels-Alder DA polymer networks. The Diels-Alder reaction is a reversible reaction. There are different ways the two original rings can combine leading to different stereochemical outcomes.
 Source: youtube.com
Source: youtube.com
Enjoy the videos and music you love upload original content and share it all with friends family and the world on YouTube. Fixed conformation in bicyclic products. The reactions were then carried out and the products analyzed and the results considered in terms of the relative energies of the reactants and products of each process and. Endo-Exo isomerism is a special type of isomerism that falls into the category of stereoisomers. Diels-Alder Transition State Benzene The diene must adopt an s-cis conformation to be reactive.
The difference between endothermic and exothermic reactions lies in the words themselves.
Generally the endo rule applies to Diels-Alder reactions but it is less adhered to than facial selectivity. Exo isomeric attachments in Diels-Alder DA polymer networks. Qualitative and quantitative MO methods were used to test the assumption that the endo-product is the predicted kinetic product and the exo-adduct is the predicted thermodynamic product for the Diels-Alder reaction of maleic anhydride with cyclopentadiene and with furan. The Diels-Alder reaction is a reversible reaction.

Topological analysis of the electron localization function ELF of dieniminiums showed that their electronic structures can been seen as the sum of those of butadiene and ethaniminium. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge. Thermic refers to heat just as in the word thermometer Exo means outside and endo means inside Thus an endothermic reaction pulls heat into an. Maleic anhydride and cyclopentadiene yield the endo product in a Diels-Alder reaction though for steric reasons the exo product is thermally the more stable one.
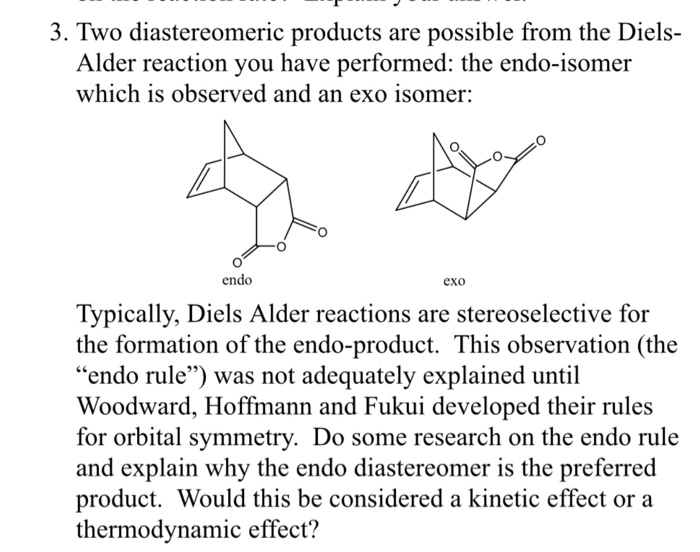
Exo isomeric attachments in Diels-Alder DA polymer networks. An exo addition looks something like this schematically. We will discuss thermodynamic and kinetic aspects of endo vs. Exo isomeric attachments in Diels-Alder DA polymer networks.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
These two outcomes are called exo and endo addition. Thermic refers to heat just as in the word thermometer Exo means outside and endo means inside Thus an endothermic reaction pulls heat into an. Exo isomeric attachments in Diels-Alder DA polymer networks. When Diels and Alder originally discovered this phenomenon they assigned the name endo to the major product where the dienophile is pointing in towards the alkene and the term exo outside such as in exoskeleton to refer to the minor product where the dienophile is pointing out away from the alkene.
The Diels-Alder reaction is a reversible reaction.
Enjoy the videos and music you love upload original content and share it all with friends family and the world on YouTube. Exo isomeric attachments in Diels-Alder DA polymer networks. Thermic refers to heat just as in the word thermometer Exo means outside and endo means inside Thus an endothermic reaction pulls heat into an. Diels-Alder Transition State Benzene The diene must adopt an s-cis conformation to be reactive. Only by heating to 200 C is the endo isomer transformed into the more stable exo isomer.
 Source: nature.com
Source: nature.com
Thermic refers to heat just as in the word thermometer Exo means outside and endo means inside Thus an endothermic reaction pulls heat into an. The exo product is more stable but the activation energy for endo is lower so the less stable endo product is formed faster. Interactive 3D animations to predict stereochemistry of endo product of Diels-Alder reaction for students studying University courses. Fixed conformation in bicyclic products. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge.
There are different ways the two original rings can combine leading to different stereochemical outcomes.
Exo -CHO in the Diels-Alder addition of a trans -αβ-unsaturated aldehyde to cyclopentadiene can be changed from 12 to 81 depending on temperature and BF 3. Qualitative and quantitative MO methods were used to test the assumption that the endo-product is the predicted kinetic product and the exo-adduct is the predicted thermodynamic product for the Diels-Alder reaction of maleic anhydride with cyclopentadiene and with furan. Fixed conformation in bicyclic products. Enjoy the videos and music you love upload original content and share it all with friends family and the world on YouTube.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. In the endo product the substituents of the dienophile are pointing towards the larger bridge while in the exo isomer they are pointing away from the larger bridge. Endo and exo isomer have different dissociation temperatures and the ratio between them plays a crucial role in harnessing DA networks thermomechanical behaviors. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. The difference between endothermic and exothermic reactions lies in the words themselves.
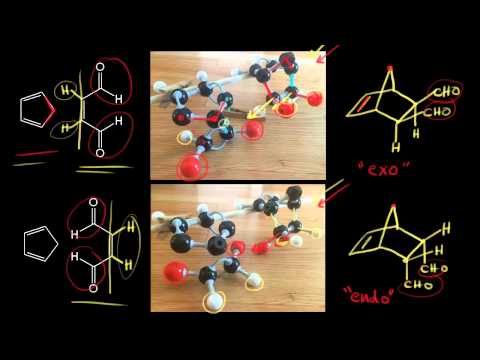 Source: khanacademy.org
Source: khanacademy.org
We will discuss thermodynamic and kinetic aspects of endo vs. We will discuss thermodynamic and kinetic aspects of endo vs. S-cis reactive conformation s-trans unreactive conformation Endo vs. In general endo is the major product because it is formed when the electron-withdrawing groups of the dienophile are pointing towards the π electrons of the diene. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level.
 Source: nature.com
Source: nature.com
An exo addition looks something like this schematically. The intramolecular ionic DielsAlder IIDA reactions of two dieniminiums were studied within the Molecular Electron Density Theory MEDT at the ωB97XD6-311Gdp computational level. The reactions were then carried out and the products analyzed and the results considered in terms of the relative energies of the reactants and products of each process and. The Diels-Alder reaction is a reversible reaction. There are different ways the two original rings can combine leading to different stereochemical outcomes.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title diels alder endo vs exo by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





